| News / Tech News |
Carbon-capture batteries developed to store renewable energy, help climate
Researchers at the Department of Energy’s Oak Ridge National Laboratory are developing battery technologies to fight climate change in two ways, by expanding the use of renewable energy and capturing airborne carbon dioxide.
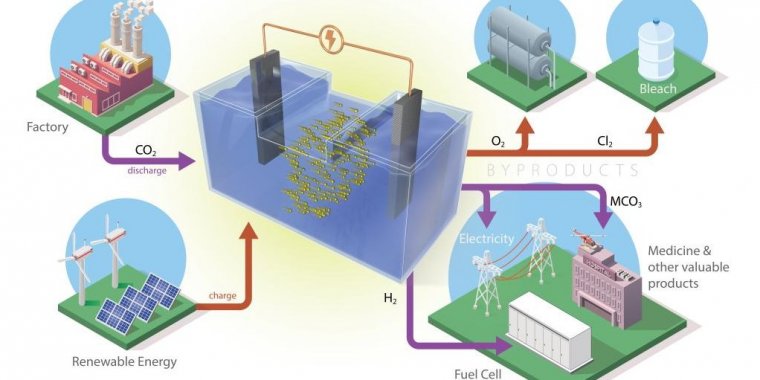
The battery developed at ORNL, consisting of two electrodes in a saltwater solution, pulls atmospheric carbon dioxide into its electrochemical reaction and releases only valuable byproducts. Photo: Andy Sproles/ORNL, U.S. Dept. of Energy
This type of battery stores the renewable energy generated by solar panels or wind turbines. Utilizing this energy when wind and sunlight are unavailable requires an electrochemical reaction that, in ORNL’s new battery formulation, captures carbon dioxide from industrial emissions and converts it to value-added products.
ORNL researchers recently created and tested two different formulations for batteries that convert carbon dioxide gas, or CO2, into a solid form that has the potential to be used in other products.
One of these new battery types maintained its capacity for 600 hours of use and could store up to 10 hours of electricity. Researchers also identified, studied and overcame the primary challenge, a deactivation caused by chemical buildup, that had been an obstacle for the other battery formulation.
Batteries operate through electrochemical reactions that move ions between two electrodes through an electrolyte. Unlike cell phone or car batteries, those designed for grid energy storage do not have to function as a portable, closed system. This allowed ORNL researchers to create and test two types of batteries that could convert CO2 from stationary, industrial sources.
For example, CO2 generated by a power plant could be pumped through a tube into the liquid electrolyte, creating bubbles similar to those in a carbonated soft drink. During battery operation, the gas bubbles turn into a solid powder.
Each component of a battery can be made of different elements or compounds. These choices determine the battery’s operational lifetime, how much energy it can store, how big or heavy it is, and how fast it charges or consumes energy. Of the new ORNL battery formulations, one combines CO2 with sodium from saltwater using an inexpensive iron-nickel catalyst. The second combines the gas with aluminum.
Each approach uses abundant materials and a liquid electrolyte in the form of saltwater, sometimes mixed with other chemicals. The batteries are safer than existing technology because their electrodes are stable in water, said lead researcher Ruhul Amin.
Very little CO2 battery research has been conducted. The previously-tried approach relies on a reversible metal-CO2 reaction that regenerates carbon dioxide, continuing to contribute greenhouse gases to the atmosphere. In addition, solid discharge products tend to clog the surface of the electrode, degrading the battery performance.
However, the CO2 batteries developed at ORNL do not release carbon dioxide. Instead, the carbonate byproduct dissolves in the liquid electrolyte.
The byproduct either continuously enriches the liquid to enhance battery performance, or it can be filtered from the bottom of the container without interrupting battery operation. Battery design can even be tuned to create more of these byproducts for use by the pharmaceutical or cement industries.
The only gases released are oxygen and hydrogen, which do not contribute to climate change and can even be captured to produce energy or fuel.
ORNL researchers used an almost completely new combination of materials for these CO2 batteries. The few similar previous designs worked for only short periods or incorporated expensive metals.
The sodium-carbon dioxide, or Na-CO2, battery was developed first and faced some obstacles.
For this system to function, the electrodes must be separated in wet and dry chambers with a solid ion conductor between them. The barrier slows the movement of ions, which in turn slows down battery operation, reducing battery efficiency.
One significant challenge for this Na-CO2 battery is that after prolonged use, a film forms on the electrode surface, which eventually causes the battery to deactivate.
Amin’s research team used highly specialized microscopes and X-ray techniques to examine the battery cell when it failed and at various stages of operation.
Studying how the film formed helped researchers understand how to break it down again. They were intrigued to realize the battery could be reactivated, or prevented from deactivating at all, simply through operational changes in the charge/discharge cycle. Uneven pulses of charging and discharging prevented film buildup on the electrode.
“We are reporting for the first time that the deactivated cell can be reactivated,” Amin said. “And we found the origin of the deactivation and activation. If you symmetrically charge-discharge the battery too long, it’s dead at one stage. If you use the protocol we established for our cell, the chance of failure is very slim.”
Next, researchers focused on the design of the aluminum-carbon dioxide, or Al-CO2, battery. The team experimented with various electrolyte solutions and three different synthesis processes to identify the best combination. The result was a battery which provides enough storage for more than 10 hours of electricity to be used later.
Testing found that the ORNL battery could operate more than 600 hours without losing capacity, Amin said – far more than the only previously reported Al-CO2 battery, which was only tested for eight hours of cycling.
This battery captures almost twice as much carbon dioxide as the Na-CO2 battery. It can be designed for the system to operate in a single chamber, with both electrodes in the same liquid solution, so there is no barrier to ion movement.
The challenge for the Al-CO2 battery is to bring it closer to scale-up, Amin said. Even so, the team will continue systematically studying its properties to extend the operating lifetime and capture CO2 more efficiently.
For the Na-CO2 battery to be competitive, the team will focus on developing a very fine, dense, mechanically stable ceramic membrane to separate the battery chambers.
YOU MAY ALSO LIKE





