| Health / Health News |
Proteins modified in lungs offer clues to biological functions of bromine
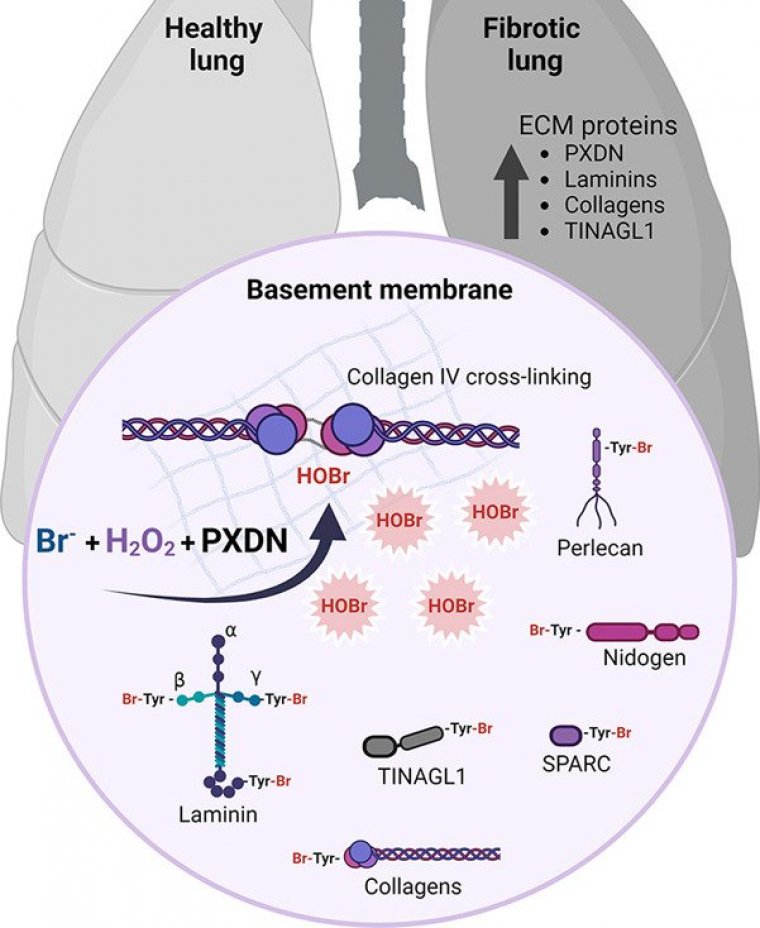
Researchers at the Center for Research on Redox Processes in Biomedicine (Redoxome) in Brazil have identified extracellular matrix proteins modified by the action of hypobromous acid in normal and fibrotic lung tissues, suggesting a possible physiological role for protein bromination.
he study identified extracellular matrix proteins modified by the action of hypobromous acid in both healthy and fibrotic lungs. Photo: Redox Biology
This is the first study to identify brominated proteins in pulmonary fibrosis and non-fibrotic lung tissue.
Redoxome is a Research, Innovation and Dissemination Center (RIDC) funded by FAPESP and hosted by the University of São Paulo’s Institute of Chemistry (IQ-USP).
The extracellular matrix is a complex three-dimensional network of proteins and other macromolecules that binds and anchors cells to form tissues, performing vital functions for cellular support, intercellular communications and regulation of tissue development.
“This research showed that bromination occurs physiologically and that peroxidasin, the enzyme that catalyzes the formation of hypobromous acid, has other targets besides collagen, which was one of the most important questions in the field,” said Litiele Cezar Cruz, first author of the article.
She conducted the research during her postdoctoral studies under the supervision of Flavia Meotti, a professor at IQ-USP and a member of Redoxome.
Halogenation is a chemical reaction in which one or more atoms of a halogen (mainly fluorine, chlorine, bromine, or iodine) is added to a compound. Halogen modifications of biological molecules are increasingly recognized in several areas of biology, but their importance in the mammalian organism has not been widely studied.
The discovery of the role of bromide ions in the oxidative cross-linking of collagen IV within the extracellular matrix was the first evidence that bromine plays an essential role in living organisms.
“We know more about chlorination, which depends on myeloperoxidase, an inflammatory enzyme. In general, this kind of disease-linked halogenation is more widely studied because inflammation is at the root of a great many diseases. Inflammatory diseases with myeloperoxidase activity tend to involve an increase in proteins with the addition of chlorine.
This modification by bromine is very similar, as chlorine and bromine are very similar chemical elements, and the modification occurs in the same amino acids, including tyrosine. However, although plasma levels of bromine are a thousand times lower than levels of chlorine, the latter is not a good substrate for peroxidasin, and more hypobromous acid than hypochlorous acid is therefore produced in the extracellular matrix,” Meotti explained.
Cruz set out to extend this knowledge by investigating the presence of bromotyrosine-modified proteins in samples of lung tissue obtained from healthy humans and patients with idiopathic pulmonary fibrosis.
She also reanalyzed published lung tissue proteomics data. In both tissue samples and proteomics data, she found modifications in several extracellular membrane proteins, even in healthy lungs. (Agência FAPESP)
YOU MAY ALSO LIKE





